Saturday, December 30, 2006
New Research on the Co-evolution of Bats and Moths
Current understanding of the co-evolution* of bats and moths has been thrown into question following new research reported in Current Biology.
Dr James Windmill from the University of Bristol has shown how the Yellow Underwing moth changes its sensitivity to a bat's calls when the moth is being chased. And in case there is another attack, the moth's ear remain tuned in for several minutes after the calls stop.
Dr Windmill said: "Because the moth cleverly tunes its ear to enhance its detection of bats, we must now question whether the bat in turn modifies its calls to avoid detection by the moth. In view of the vast diversity of bat calls, this is only to be expected.
"To date, this phenomenon has not been reported for insects or, in fact, for any other hearing system in the animal kingdom. These findings change our understanding of the co-evolution of bats and moths and have implications for the hearing of many other animals."
It has been known for over 50 years that moths can hear the ultrasonic hunting calls of their nocturnal predator, the bat. Previously it was thought that these ears were only partially sensitive to the sound frequencies commonly used by bats and that bats would make their hunting calls inaudible to moths.
But now it appears that even though moth ears are among the simplest in the insect world - they have only two or four vibration sensitive cells attached to a small eardrum - moths are not as deaf as previously thought.
As a bat gets closer to the moth, both the loudness and frequency (pitch) of the bat's calls increase. Surprisingly, the sensitivity of the moth's ear to the bat's calls also increases. This occurs because the moth's ear dynamically becomes more sensitive to the frequencies that many bats use when attacking moths.
This multidisciplinary work involved engineers, biologists and physicists; biological measurements are accompanied by a mathematical model explaining the basis for the unconventional behaviour of the moth's ear.
Original Press Release ("How to avoid a bat" - 19th December 2006) available via this link.
-------
Based on the paper:
Keeping up with Bats: Dynamic Auditory Tuning in a Moth
James Frederick Charles Windmill1, Joseph Curt Jackson, Elizabeth Jane Tuck and Daniel Robert
Many night-flying insects evolved ultrasound sensitive ears in response to acoustic predation by echolocating bats. Noctuid moths are most sensitive to frequencies at 20-40 kHz, the lower range of bat ultrasound. This may disadvantage the moth because noctuid-hunting bats in particular echolocate at higher frequencies shortly before prey capture and thus improve their echolocation and reduce their acoustic conspicuousness. Yet, moth hearing is not simple; the ear's nonlinear dynamic response shifts its mechanical sensitivity up to high frequencies. Dependent on incident sound intensity, the moth's ear mechanically tunes up and anticipates the high frequencies used by hunting bats. Surprisingly, this tuning is hysteretic, keeping the ear tuned up for the bat's possible return. A mathematical model is constructed for predicting a linear relationship between the ear's mechanical stiffness and sound intensity. This nonlinear mechanical response is a parametric amplitude dependence that may constitute a feature common to other sensory systems. Adding another twist to the coevolutionary arms race between moths and bats, these results reveal unexpected sophistication in one of the simplest ears known and a novel perspective for interpreting bat echolocation calls.
-------
*Info on co-evolution:
In biology, co-evolution is the mutual evolutionary influence between two species. Each party in a co-evolutionary relationship exerts selective pressures on the other, thereby affecting each others' evolution. Co-evolution includes the evolution of a host species and its parasites, in examples of mutualism evolving through time. Few perfectly isolated examples of evolution can be identified. Evolution in response to abiotic factors, such as climate change, is not coevolution (since climate is not alive and does not undergo biological evolution). Evolution in a one-on-one interaction, such as that between a specialized host-symbiont or host-parasite pair, is coevolution. But many cases are less clearcut: a species may evolve in response to a number of other species, each of which is also evolving in response to a set of species. This situation has been referred to as "diffuse coevolution". And, certainly, for many organisms, the biotic (living) environment is the most prominent selective pressure, resulting in evolutionary change.
Technorati: co-evolution, bats, moths, new, research, current, biology, university, bristol, moth, sensitivity, ear, detection, diversity, phenomenon, hearing, animal, kingdom, vibration, ultrasonic, echolocation, evolution, climate, change, selective, pressure
Add to: CiteUlike | Connotea | Del.icio.us | Digg | Furl | Newsvine | Reddit | Yahoo
Friday, December 29, 2006
The Eocene Epoch: Ancient insects used advanced camouflage
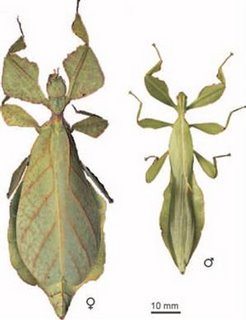
A fossil of a leaf-imitating insect from 47 million years ago bears a striking resemblance to the mimickers of today.
The discovery represents the first fossil of a leaf insect (Eophyllium messelensis), and also shows that leaf imitation is an ancient and successful evolutionary strategy that has been conserved over a relatively long period of time.
Scientists led by Sonja Wedmann of the Institute of Paleontology in Bonn, Germany, unearthed the remains at a well-known fossil site called Messel*, in Hessen, Germany.
The 2.4-inch-long insect had physical characteristics similar to the oblong leaves of trees living there at the time, including Myrtle trees, legumes, such as alfalfa, and Laurel trees.
Continued at "Ancient insects used advanced camouflage" [The Eocene Epoch**] [Image: PNAS]
-------
Based on the Proceedings of the National Academy of Sciences (PNAS) paper:
The first fossil leaf insect: 47 million years of specialized cryptic morphology and behavior
Sonja Wedmann, Sven Bradler, and Jes Rust
Published online before print December 29, 2006, 10.1073/pnas.0606937104
PNAS | January 9, 2007 | vol. 104 | no. 2 | 565-569
Abstract
Stick and leaf insects (insect order Phasmatodea) are represented primarily by twig-imitating slender forms. Only a small percentage (approx 1%) of extant phasmids belong to the leaf insects (Phylliinae), which exhibit an extreme form of morphological and behavioral leaf mimicry. Fossils of phasmid insects are extremely rare worldwide. Here we report the first fossil leaf insect, Eophyllium messelensis gen. et sp. nov., from 47-million-year-old deposits at Messel in Germany. The new specimen, a male, is exquisitely preserved and displays the same foliaceous appearance as extant male leaf insects. Clearly, an advanced form of extant angiosperm leaf mimicry had already evolved early in the Eocene. We infer that this trait was combined with a special behavior, catalepsy or "adaptive stillness," enabling Eophyllium to deceive visually oriented predators. Potential predators reported from the Eocene are birds, early primates, and bats. The combination of primitive and derived characters revealed by Eophyllium allows the determination of its exact phylogenetic position and illuminates the evolution of leaf mimicry for this insect group. It provides direct evidence that Phylliinae originated at least 47 Mya. Eophyllium enlarges the known geographical range of Phylliinae, currently restricted to southeast Asia, which is apparently a relict distribution. This fossil leaf insect bears considerable resemblance to extant individuals in size and cryptic morphology, indicating minimal change in 47 million years. This absence of evolutionary change is an outstanding example of morphological and, probably, behavioral stasis.
Also see:
Correction for Wedmann et al., PNAS 104 (2) 565-569.
Published online before print January 19, 2007, 10.1073/pnas.0700092104
PNAS | February 6, 2007 | vol. 104 | no. 6 | 2024
-------
*Info on the Messel Pit Fossil Site:
"...The Messel fossil finds are extraordinary in more ways than one: entire skeletons are preserved perfectly here - birds with their feathers, mammals with skin and hair. They provide evidence of an important period in the evolutionary history of mammals, which were able to develop at a rapid rate after the extinction of the dinosaurs.
The "Messel Propalaeotherium" represents an early European side branch of the horse family tree: they were much smaller than horses today, lived in the rainforest undergrowth and fed on foliage and fruit, as was established from their teeth and stomach contents. The discovery of an anteater was a huge surprise for palaeontologists, since it originally came from South America. The most common mammal finds are the bats, with the seven species that existed in those times occupying various ecological niches.
The Messel Lake also preserved an astonishingly large number of birds, various reptiles such as turtles, crocodiles and snakes, as well as fish, frogs and a diverse insect fauna. There are 60 families of flora, including various plants whose relations thrive today in South-east Asia, Central and South America. Botanical details have been well-preserved too: flowers in which the pollen grains have survived, as well as fruit clusters and individual fruits..."
-------
**Info on The Eocene Epoch:
"The Eocene epoch is part of the Tertiary Period in the Cenozoic Era, and lasted from about 54.8 to 33.7 million years ago (mya). The oldest known fossils of most of the modern orders of mammals appear in a brief period during the Early Eocene and all were small, under 10 kg. Both groups of modern ungulates (Artiodactyla and Perissodactyla) became prevalent mammals at this time, due to a major radiation between Europe and North America."
-------
A recent post on mimicry: "Predator Mimicry: Metalmark Moths Mimic Their Jumping Spider Predators"
Technorati: fossil, leaf, insect, discovery, imitation, ancient, evolutionary, strategy, paleontology, institute, bonn, messil, site, germany, hessen, insects, camouflage, eocene, epoch, pnas, trait, fossils, mimicry, evolution, change, pit, history, mammals, extinction, dinosaurs
Add to: CiteUlike | Connotea | Del.icio.us | Digg | Furl | Newsvine | Reddit | Yahoo
How many genes does it take to learn? Lessons from sea slugs
At any given time within just a single brain cell of sea slug known as Aplysia, more than 10,000 genes are active, according to scientists writing in Friday's (December 29, 2006) edition of the journal Cell. The findings suggest that acts of learning or the progression of brain disorders do not take place in isolation - large clusters of genes within an untold amount of cells contribute to major neural events.
'For the first time we provide a genomic dissection of the memory-forming network,' said Leonid Moroz*, a professor of neuroscience and zoology at the University of Florida Whitney Laboratory for Marine Bioscience. 'We took advantage of this powerful model of neurobiology and identified thousands of genes operating within a single neuron. Just during any simple event related to memory formation, we expect differences in gene expression for at least 200 to 400 genes.'
Researchers studied gene expression in association with specific networks controlling feeding or defensive reflexes in the sea slug. To their surprise, they identified more than 100 genes similar to those associated with all major human neurological diseases and more than 600 genes controlling development, confirming that molecular and genomic events underlying key neuronal functions were developed in early animal ancestors and remained practically unchanged for more than 530 million years of independent evolution in the lineages leading to men or sea slugs.
Moroz and his collaborators uncovered new information that suggest that gene loss in the evolution of the nervous system is as important as gene gain in terms of adaptive strategies. They believe that a common ancestor of animals had a complex genome and different genes controlling brain or immune functions were lost independently in different lineages of animals, including humans.
Until now, scientists have been largely in the dark about how genes control the generation of specific brain circuitry and how genes modify that circuitry to enable learning and memory. For that matter, little is known about the genes that distinguish one neuron from the next, even though they may function quite differently.
Molecular analyses of Aplysia neuronal genes are shedding light on these elusive processes. In 2000, senior author Eric Kandel, M.D., of Columbia University in New York shared the Nobel Prize in Physiology or Medicine for his work using Aplysia as a model of how memories are formed in the human brain.
Despite its simple nervous system - Aplysia has about 10,000 large neurons that can be easily identified, compared with about one hundred billion neurons in humans - the animal is capable of learning and its brain cells communicate in many ways identical to human neural communication.
In the new findings, scientists identified more than 175,000 gene tags useful for understanding brain functions, increasing by more than 100 times the amount of genomic information available for study, according to Moroz and 22 other researchers from UF and Columbia University. More than half of the genes have clear counterparts in humans and can be linked to a defined neuronal circuitry, including a simple memory-forming network.
"In the human brain there are a hundred billion neurons, each expressing at least 18,000 genes, and the level of expression of each gene is different," said Moroz, who is affiliated with UF's Evelyn F. and William L. McKnight Brain Institute and the UF Genetics Institute. "Understanding individual genes or proteins is important, but this is a sort of molecular alphabet. This helps us learn the molecular grammar, or a set of rules that can control orchestrated activity of multiple genes in specific neurons. If we are going to understand memory or neurological disease at the cellular level, we need to understand the rules."
Scientists also analyzed 146 human genes implicated in 168 neurological disorders, including Parkinson's and Alzheimer's diseases, and genes controlling aging and stem-cell differentiation. They found 104 counterpart genes in Aplysia, suggesting it will be a valuable tool for developing treatments for neurodegenerative diseases.
"The authors have assembled a tremendous amount of data on gene transcripts associated with neuronal signaling pathways in Aplysia that sheds new light on evolutionary relationships of this very ancient and highly successful marine animal," said Dennis Steindler, Ph.D., executive director of UF's McKnight Brain Institute, who did not participate in the research. "A very important part of this study is the discovery of novel genes not formerly associated with the mollusk genome that include many associated with neurological disorders."
The findings are especially important for scientists using mollusks in experimental systems, according to Edgar Walters, Ph.D., a professor of integrative biology and pharmacology at the University of Texas Medical School at Houston, who was not involved in the research.
"Few animals other than Aplysia allow scientists to relate a molecular pathway directly to the function of a cell, all in context with an animal's behavior," Walters said. "In a mammal, it's hard to identify and manipulate a single cell and know what its function is. With Aplysia, there is direct access to whatever cell you're interested in with just a micropipette. As a scientist who wants to know which molecules are present in Aplysia for experimental manipulation, I am very happy to see this paper come out."
Source: University of Florida
-------
Based on the paper:
Neuronal Transcriptome of Aplysia: Neuronal Compartments and Circuitry
By Leonid L. Moroz et al.
Cell, Vol 127, 1453-1467, 29 December 2006
Summary
Molecular analyses of Aplysia, a well-established model organism for cellular and systems neural science, have been seriously handicapped by a lack of adequate genomic information. By sequencing cDNA libraries from the central nervous system (CNS), we have identified over 175,000 expressed sequence tags (ESTs), of which 19,814 are unique neuronal gene products and represent 50%-70% of the total Aplysia neuronal transcriptome. We have characterized the transcriptome at three levels: (1) the central nervous system, (2) the elementary components of a simple behavior: the gill-withdrawal reflex - by analyzing sensory, motor, and serotonergic modulatory neurons, and (3) processes of individual neurons. In addition to increasing the amount of available gene sequences of Aplysia by two orders of magnitude, this collection represents the largest database available for any member of the Lophotrochozoa and therefore provides additional insights into evolutionary strategies used by this highly successful diversified lineage, one of the three proposed superclades of bilateral animals.
-------
*Info from Leonid Moroz's webpage:
Our laboratory works to characterize basic mechanisms underlying the design of nervous systems and evolution of neuronal signaling mechanisms. The major questions are: (1) why are individual neurons so different from each other, (2) how do they maintain such precise connections between each other, (3) how does this fixed wiring result in such enormous neuronal plasticity and (4) how does this contribute to learning and memory mechanisms? By taking advantage of relatively simpler nervous systems of invertebrate animals as models, we conbine neuroscience,genomics, bioinformatics, evolutionary theory, zoology, molecular biology, microanalytical chemistry and nanoscience to understand how neurons operate, remember and learn.
Technorati: brain, cell, sea slug, aplysia, genes, journal, learning, disorders, neural, memory, zoology, marine, bioscience, neurobiology, nervous, system, genome, circuitry, common, ancestor, central, behavior, evolution
Add to: CiteUlike | Connotea | Del.icio.us | Digg | Furl | Newsvine | Reddit | Yahoo
Thursday, December 28, 2006
Developmental Biology: Special Issue on the Sea Urchin Genome
From the journal Developmental Biology: Free online access to Volume 300 Issue 1, Sea Urchin Genome: Implications and Highlights up until end of June for non-subscribers.
From Eric Davidson's* introductory paper "Special issue: The sea urchin genome":
The Strongylocentrotus purpuratus Genome Project focused the attention of the sea urchin research community as nothing had ever done before. Two numbers tell the story. The first is the more than 9700 genes annotated by volunteers from this research community, guided by the energetic leadership of Erica Sodergren and George Weinstock at the Baylor College of Medicine Human Genome Sequencing Center, where the sequence was obtained and the annotation effort was organized. The second is the number of papers in this very issue, which contains 36 individual studies no one of which could or would have existed absent the genome sequence. Together with the main announcement of the genome sequence in Science and four additional genome-related papers published with it, over 40 diverse works have been called into existence with the advent of this sequence. The genome sequence provides a digital definition of the potentialities of the animal, and these papers show how many different kinds of potentiality it illuminated. This collection contains remarkable surprises, and some of the papers herein literally set up new fields of scientific enterprise...
...The deuterostomes were first imagined a century ago on the basis of comparative embryo anatomy, perhaps the greatest early success story of that field; their reality as a clade was indicated by pre-genomic evidence such as intron position in shared genes, then strongly supported by rRNA and protein molecular phylogeny. But now this superphylum, our own, is defined by the sea urchin genome project in terms of the sharing patterns of literally thousands of genes. The other side of the coin is the gene families that appeared or have hugely expanded during echinoderm evolution, most prominently the sensory receptor genes, immune genes of several large families, and the biomineralization genes, which are unlike any seen elsewhere. It is no wonder that there are differences and surprises: this is also the first non-chordate marine genome to be sequenced, the first sequence of a maximum indirectly developing animal, as well as the first echinoderm genome...
See Special issue: The sea urchin genome - Table of Contents
-------
Papers include:
1) Shedding genomic light on Aristotle's lantern
Erica Sodergren, Yufeng Shen, Xingzhi Song, Lan Zhang, Richard A. Gibbs, George M. Weinstock
Abstract
Sea urchins have proved fascinating to biologists since the time of Aristotle who compared the appearance of their bony mouth structure to a lantern in The History of Animals. Throughout modern times it has been a model system for research in developmental biology. Now, the genome of the sea urchin Strongylocentrotus purpuratus is the first echinoderm genome to be sequenced. A high quality draft sequence assembly was produced using the Atlas assembler to combine whole genome shotgun sequences with sequences from a collection of BACs selected to form a minimal tiling path along the genome. A formidable challenge was presented by the high degree of heterozygosity between the two haplotypes of the selected male representative of this marine organism. This was overcome by use of the BAC tiling path backbone, in which each BAC represents a single haplotype, as well as by improvements in the Atlas software. Another innovation introduced in this project was the sequencing of pools of tiling path BACs rather than individual BAC sequencing. The Clone-Array Pooled Shotgun Strategy greatly reduced the cost and time devoted to preparing shotgun libraries from BAC clones. The genome sequence was analyzed with several gene prediction methods to produce a comprehensive gene list that was then manually refined and annotated by a volunteer team of sea urchin experts. This latter annotation community edited over 9000 gene models and uncovered many unexpected aspects of the sea urchin genetic content impacting transcriptional regulation, immunology, sensory perception, and an organism's development. Analysis of the basic deuterostome genetic complement supports the sea urchin's role as a model system for deuterostome and, by extension, chordate development.
2) High regulatory gene use in sea urchin embryogenesis: Implications for
bilaterian development and evolution
Meredith Howard-Ashby, Stefan C. Materna, C. Titus Brown, Qiang Tu, Paola Oliveri,
R. Andrew Cameron, Eric H. Davidson
A global scan of transcription factor usage in the sea urchin embryo was carried out in the context of the Strongylocentrotus purpuratus genome sequencing project, and results from six individual studies are here considered. Transcript prevalence data were obtained for over 280 regulatory genes encoding sequence-specific transcription factors of every known family, but excluding genes encoding zinc finger proteins. This is a statistically inclusive proxy for the total “regulome” of the sea urchin genome. Close to 80% of the regulome is expressed at significant levels by the late gastrula stage. Most regulatory genes must be used repeatedly for different functions as development progresses. An evolutionary implication is that animal complexity at the stage when the regulome first evolved was far simpler than even the last common bilaterian ancestor, and is thus of deep antiquity.
-------
*From Eric Davidson's lab homepage:
"...The major focus of research in our laboratory is on gene networks that control development and their evolution. Our areas of research include the transcriptional mechanisms by which specification of embryonic blastomeres occurs early in development; structure/function relationships in developmental cis-regulatory systems; sea urchin genomics; and regulatory evolution in the bilaterians. Most of our work is carried out on sea urchin embryos, which provide key experimental advantages..."
Technorati: developmental, biology, sea urchin, genome, implications, highlights, project, research, human, science, anatomy, evolution, genes, aristotle, lantern, genetic, immunology, evolutionary, complexity, networks
Add to: CiteUlike | Connotea | Del.icio.us | Digg | Furl | Newsvine | Reddit | Yahoo
Wednesday, December 27, 2006
The Syntax and Meaning of Wild Gibbon Songs
An open access/free paper from PLoS ONE:
The Syntax and Meaning of Wild Gibbon Songs
By Esther Clarke, Ulrich H. Reichard, Klaus Zuberbuhler*
Spoken language is a result of the human capacity to assemble simple vocal units into more complex utterances, the basic carriers of semantic information. Not much is known about the evolutionary origins of this behaviour. The vocal abilities of non-human primates are relatively unimpressive in comparison, with gibbon songs being a rare exception. These apes assemble a repertoire of call notes into elaborate songs, which function to repel conspecific intruders, advertise pair bonds, and attract mates. We conducted a series of field experiments with white-handed gibbons** at Khao Yai National Park, Thailand, which showed that this ape species uses songs also to protect themselves against predation. We compared the acoustic structure of predatory-induced songs with regular songs that were given as part of their daily routine. Predator-induced songs were identical to normal songs in the call note repertoire, but we found consistent differences in how the notes were assembled into songs. The responses of out-of-sight receivers demonstrated that these syntactic differences were meaningful to conspecifics. Our study provides the first evidence of referential signalling in a free-ranging ape species, based on a communication system that utilises combinatorial rules.
Citation: Clarke E, Reichard UH, Zuberbühler K (2006) The Syntax and Meaning of Wild Gibbon Songs. PLoS ONE 1(1): e73. doi:10.1371/journal.pone.0000073
Continued at "The Syntax and Meaning of Wild Gibbon Songs" [Primatology]
-------
*Klaus Zuberbuhler is author of:
The Phylogenetic Roots of Language - Evidence From Primate Communication and Cognition
The anatomy of the nonhuman primate vocal tract is not fundamentally different from the human one. Notwithstanding, nonhuman primates are remarkably unskillful at controlling vocal production and at combining basic call units into more complex strings. Instead, their vocal behavior is linked to specific psychological states, which are evoked by events in their social or physical environment. Humans are the only primates that have evolved the ability to produce elaborate and willfully controlled vocal signals, although this may have been a fairly recent invention. Despite their expressive limitations, nonhuman primates have demonstrated a surprising degree of cognitive complexity when responding to other individuals' vocalizations, suggesting that, as recipients, crucial linguistic abilities are part of primate cognition. Pivotal aspects of language comprehension, particularly the ability to process semantic content, may thus be part of our primate heritage. The strongest evidence currently comes from Old World monkeys, but recent work indicates that these capacities may also be present in our closest relatives, the chimpanzees.
And co-author (with Kate Arnold) of:
Language evolution: Semantic combinations in primate calls
Syntax sets human language apart from other natural communication systems, although its evolutionary origins are obscure. Here we show that free-ranging putty-nosed monkeys combine two vocalizations into different call sequences that are linked to specific external events, such as the presence of a predator and the imminent movement of the group. Our findings indicate that non-human primates can combine calls into higher-order sequences that have a particular meaning.
-------
**Info on white-handed gibbons:
...The white-handed gibbon is distinguished by its musical howl. They are quiet during the day but commonly howl at sunrise and sunset. They are very vocal, making loud "whoop" sounds. Their loud resonant songs can be heard up to 1/2 mile away. Songs by far excel those of all other species because of a sound-amplifying throat sac.
Duetting is the singing between the male and female, and is dominated by the female. This helps to maintain the pair bond between the pair and to maintain the territory. Each morning upon awakening a family group of gibbons loudly announces its presence in the forest, using a territorial hooting call and menacing gestures. This call warns other gibbons to stay out of their territory (and especially away from the local fruit trees). This noisy display takes 1/2 hour or more every morning and is usually started by the adult female. The male and female have different calls.
In friendly greetings, corners of mouth are drawn back, revealing teeth, and tongue is sometimes protruding. In anger, mouth is opened and closed repeatedly, smacking lips and snapping teeth together. Snarling is interpreted as an intention of biting.
There are 9 species with 9 different territorial songs. The gibbons seem to be born knowing the songs because they are always the same, and not learned...
See "Sexual Selection and the Evolution of Brain Size in Primates"
Technorati: syntax, meaning, white, handed, gibbons, gibbon, songs, language, evolutionary, origins, apes, primates, primatology, species, study, anatomy, humans, monkeys, old world, semantic, primate, evolution, complexity
Add to: CiteUlike | Connotea | Del.icio.us | Digg | Furl | Newsvine | Reddit | Yahoo
The discordant eardrum (PNAS)
An open access/free article from the Proceedings of the National Academy of Sciences (PNAS):
by Jonathan P. Fay, Sunil Puria*, and Charles R. Steele**
Edited by Eric I. Knudsen***, Stanford University School of Medicine, Stanford, California
Abstract
At frequencies above 3 kHz, the tympanic membrane vibrates chaotically. By having many resonances, the eardrum can transmit the broadest possible bandwidth of sound with optimal sensitivity. In essence, the eardrum works best through discord. The eardrum's success as an instrument of hearing can be directly explained through a combination of its shape, angular placement, and composition. The eardrum has a conical asymmetrical shape, lies at a steep angle with respect to the ear canal, and has organized radial and circumferential collagen fiber layers that provide the scaffolding. Understanding the role of each feature in hearing transduction will help direct future surgical reconstructions, lead to improved microphone and loudspeaker designs, and provide a basis for understanding the different tympanic membrane structures across species. To analyze the significance of each anatomical feature, a computer simulation of the ear canal, eardrum, and ossicles was developed. It is shown that a cone-shaped eardrum can transfer more force to the ossicles than a flat eardrum, especially at high frequencies. The tilted eardrum within the ear canal allows it to have a larger area for the same canal size, which increases sound transmission to the cochlea. The asymmetric eardrum with collagen fibers achieves optimal transmission at high frequencies by creating a multitude of deliberately mistuned resonances. The resonances are summed at the malleus attachment to produce a smooth transfer of pressure across all frequencies. In each case, the peculiar properties of the eardrum are directly responsible for the optimal sensitivity of this discordant drum.
Opening paragraphs:
The function of the middle ear in terrestrial mammals is to transfer acoustic energy between the air of the ear canal to the fluid of the inner ear. The first and crucial step of the transduction process takes place at the tympanic membrane, which converts sound pressure in the ear canal into vibrations of the middle ear bones. Understanding how the tympanic membrane manages this task so successfully over such a broad range of frequencies has been a subject of research since Helmholtz's publication in 1868 (1, 2).
Even though the function of the eardrum is clear and the anatomy of the eardrum is well characterized, the connection between the anatomical features and the ability of the eardrum to transduce sound has been missing. The missing structure-function relationships can be summarized by the following three questions. Why does the mammalian eardrum have its distinctive conical and toroidal shape? What is the advantage of its angular placement in the ear canal? What is the significance of its highly organized radial and circumferential fibers?
The shape of the human and feline eardrum is known from detailed Moire interferometry contour maps (refs. 3 and 4 and Fig. 1a). From the contour maps, three-dimensional reconstructions reveal the striking similarity of the two eardrums. In both cases, the eardrum has an elliptical outer boundary, whereas the central portion has a distinctive conical shape (Fig. 1b). As one moves away from the center, the cone starts to bend forming an outer toroidal region (Fig. 1 b and c).
-------
*Info on Sunil Puria:
...Of the five senses, the auditory system is one of the most remarkable. It can operate over a dynamic range of more than six orders of magnitude in sound pressure level. To accomplish this task, the hair cells in the fluid-filled inner ear detect motions down to the dimensions of atoms and are limited only by Brownian motion of the surrounding fluid.
It has recently been discovered that these hair cells, which act as transducers of mechanical motion to electrical impulses transmitted to the central nervous system, are inherently non-linear. Consequently, the mechanics of a normal inner-ear must remain non-linear for normal function while a damaged ear exhibits more linear characteristics. Thus the auditory system uses non-linear elements to achieve exquisite sensitivity and a large dynamic range. These non-linearities of the ear are increasingly being exploited in speech coding technologies.
Recently, it was discovered that a healthy ear not only detects sounds but also generates sounds... (More)
-------
**Info on Charles Steele:
...Asymptotic analysis and computation, biomechanics, mechanics of hearing, noninvasive mechanical measurement of bone and soft tissue, plant morphogenesis. He is the author of over 80 archival papers and three handbook chapters in these areas. He is the Editor-in-Chief of the International Journal of Solids and Structures... (more)
------
***Info on Eric Knudsen:
We study mechanisms of attention, learning and strategies of information processing in the central auditory system of developing and adult barn owls, using neurophysiological, pharmacological, anatomical and behavioral techniques. Studies focus on the process of sound localization. Sound localization is shaped powerfully by an animal's auditory and visual experience. Experiments are being conducted to elucidate developmental influences, extent and time course of this learning process, and its dependence on visual feedback... (More)
-------Also see "Origin of the vertebrate inner ear: evolution and induction of the otic placode"
by Andrea Streit (homepage)
Abstract
The vertebrate inner ear forms a highly complex sensory structure responsible for the detection of sound and balance. Some new aspects on the evolutionary and developmental origin of the inner ear are summarised here.
Recent molecular data have challenged the longstanding view that special sense organs such as the inner ear have evolved with the appearance of vertebrates. In addition, it has remained unclear whether the ear originally arose through a modification of the amphibian mechanosensory lateral line system or whether both evolved independently.
A comparison of the developmental mechanisms giving rise to both sensory systems in different species should help to clarify some of these controversies. During embryonic development, the inner ear arises from a simple epithelium adjacent to the hindbrain, the otic placode, that is specified through inductive interactions with surrounding tissues.
This review summarises the embryological evidence showing that the induction of the otic placode is a multistep process which requires sequential interaction of different tissues with the future otic ectoderm and the recent progress that has been made to identify some of the molecular players involved.
Finally, the hypothesis is discussed that induction of all sensory placodes initially shares a common molecular pathway, which may have been responsible to generate an 'ancestral placode' during evolution.
Technorati: open access, pnas, discordant, eardrum, stanford, tympanic, membrane, sound, bandwidth, ear, canal, cochlea, malleus, middle, pressure, anatomy, auditory, system, inner, vertebrates, balance, sensory, ancestral, evolution
Add to: CiteUlike | Connotea | Del.icio.us | Digg | Furl | Newsvine | Reddit | Yahoo
How does a zebrafish grow a new tail?
If a zebrafish loses a chunk of its tail fin, it'll grow back within a week. Like lizards, newts, and frogs, a zebrafish can replace surprisingly complex body parts. A tail fin, for example, has many different types of cells and is a very intricate structure. It is the fish version of an arm or leg.
The question of how cold-blooded animals re-grow missing tails and other appendages has fascinated veterinary and medical scientists. They also wonder if people, and other warm-blooded animals that evolved from these simpler creatures, might still have untapped regenerative powers hidden in their genes.
People are constantly renewing blood components, skeletal muscles and skin. We can regenerate liver tissue and repair minor injuries to bone, muscle, the tips of our toes and fingers, and the corneas of our eyes. Finding out more about the remarkable ability of amphibians and fish to re-grow complex parts might provide the information necessary to create treatments for people whose hearts, spinal cords, eyes or arms and legs have been badly hurt.
Scientists have discovered some of the genes and cell-to-cell communication pathways that enable zebrafish to restore their tail fins.
'The ability to regenerate body parts such as those that are damaged by injury or disease,' said Dr. Randall Moon*, professor of pharmacology at the University of Washington (UW), an investigator of the Howard Hughes Medical Institute, and a researcher in the UW Institute for Stem Cell and Regenerative Medicine, 'involves creating cells that can take any number of new roles. This can be done by re-programming cells that already have a given function or by activating resident stem cells.'
Continued at "How does a zebrafish grow a new tail?"
-------
Based on the journal Development paper:
Distinct Wnt signaling pathways have opposing roles in appendage regeneration
Cristi L. Stoick-Cooper, Gilbert Weidinger, Kimberly J. Riehle, Charlotte Hubbert, Michael B. Major, Nelson Fausto, and Randall T. Moon
Abstract
In contrast to mammals, lower vertebrates have a remarkable capacity to regenerate** complex structures damaged by injury or disease. This process, termed epimorphic regeneration, involves progenitor cells created through the reprogramming of differentiated cells or through the activation of resident stem cells. Wnt/beta-catenin signaling regulates progenitor cell fate and proliferation during embryonic development and stem cell function in adults, but its functional involvement in epimorphic regeneration has not been addressed. Using transgenic fish lines, we show that Wnt/beta-catenin signaling is activated in the regenerating zebrafish tail fin and is required for formation and subsequent proliferation of the progenitor cells of the blastema. Wnt/beta-catenin signaling appears to act upstream of FGF signaling, which has recently been found to be essential for fin regeneration. Intriguingly, increased Wnt/beta-catenin signaling is sufficient to augment regeneration, as tail fins regenerate faster in fish heterozygous for a loss-of-function mutation in axin1, a negative regulator of the pathway. Likewise, activation of Wnt/beta-catenin signaling by overexpression of wnt8 increases proliferation of progenitor cells in the regenerating fin. By contrast, overexpression of wnt5b (pipetail) reduces expression of Wnt/beta-catenin target genes, impairs proliferation of progenitors and inhibits fin regeneration. Importantly, fin regeneration is accelerated in wnt5b mutant fish. These data suggest that Wnt/beta-catenin signaling promotes regeneration, whereas a distinct pathway activated by wnt5b acts in a negative-feedback loop to limit regeneration.
-------
*Info on Randall Moon:
Randall Moon studies the biochemistry of the Wnt signal transduction pathways and the roles of these pathways in vertebrates. He is interested in understanding the normal mechanisms and functions of Wnt signaling and in using this understanding to develop insights into the roles of Wnt signaling in diseases. He is also interested in developing potential therapies, with an emphasis on regenerative medicine.
-------
**Info on Regeneration:
"...Regeneration occurs in many, if not all vertebrate embryos, and is present in some adult animals such as salamanders ( e.g. the newt and axolotl), hydra, horseshoe crabs and a type of mouse. [1] [2]. Mammals exhibit limited regenerative abilities, although not as impressive as salamanders. Examples of mammalian regeneration include antlers, finger tips and holes in ears. Finger tip regeneration has been well characterized, and these studies have resulted in the first demonstration of a genetic pathway controlling regeneration in a mammal. Several species of mammals can regenerate ear holes; a phenomenon that has been most studied in the MRL mouse. If the processes behind regeneration are fully understood, it is believed this would lead to better treatment for individuals with nerve injuries (such as those resulting from a broken back or a polio infection), missing limbs, and/or damaged or destroyed organs.
Regeneration of a lost limb occurs in two major steps, first de-differentiation of adult cells into a stem cell state similar to embryonic cells and second, development of these cells into new tissue more or less the same way it developed the first time [7]. Some animals like planarians instead keep clusters of non-differentiated cells within their bodies, which migrate to the parts of the body that need healing..."
Technorati: zebrafish, lizards, newts, frogs, fish, evolved, liver, bone, muscle, regenerate, amphibians, stem, cells, development, regeneration, body, parts, mutation, biochemistry, genetic, mammals, crabs, mouse, salamanders
Add to: CiteUlike | Connotea | Del.icio.us | Digg | Furl | Newsvine | Reddit | Yahoo
Tuesday, December 26, 2006
Humans Responsible for Australian Extinctions
The mystery of what killed Australia's giant animals - the so-called 'megafauna' - during the Last Ice Age is one of the longest-running and most emotive debates in palaeontology. Scientists have now published clear evidence from south-eastern Australia to show that climate change was not the driving force behind the extinctions, which took place between 50 and 40 thousand years ago.
This refocuses attention on humans as the main cause. The latest study, published in the January 2007 issue of the respected international journal Geology, is unique in providing - for the first time - a long-term perspective on the responses of the megafauna in the Naracoorte Caves (info) region of south-eastern Australia to cyclical swings in Ice Age climates.
"Climate change was certainly not the main culprit in the extinctions. Our data show that the megafauna was resilient to climatic fluctuations over the past half-million years", said team leader and palaeontologist Dr Gavin Prideaux from the Western Australian Museum and Flinders University.
Australia lost 90% of its large fauna, including rhino-sized marsupials, 3-metre tall kangaroos and giant goannas within 20 thousand years of human arrival. Opinions are divided between the relative importance of climatic changes and the activities of humans themselves via habitat disturbance or over-hunting. Unfortunately, the debate has been hamstrung by a lack of basic data on how communities responded to climate changes before humans arrived.
The new fossil evidence from Naracoorte reveals surprising stability in the mammal composition through successive wet and dry phases. "Although populations fluctuated locally in concert with cyclical climatic changes, with larger species favoured in wetter times, most if not all of them survived even the driest times - then humans arrived", said Dr Prideaux.
The Naracoorte Caves World Heritage Area in south-eastern South Australia contains the richest assemblage of Pleistocene (1.8 million to 10 thousand years ago) animals anywhere in Australia. What makes the record more remarkable is that it can be directly compared to a 500 thousand-year record of local rainfall preserved in the stalagmites in these caves.
The fossils were dated by two independent methods (optically stimulated luminescence and uranium-series dating) at the Universities of Wollongong and Melbourne, by geochronologists Professor Richard 'Bert' Roberts, Dr Kira Westaway and Dr John Hellstrom. The multi-disciplinary team also included Dr Dirk Megirian from the Museum of Central Australia in Alice Springs, who studied the sediments for additional clues of the prevailing climate conditions.
"These analyses have allowed us to pinpoint the ages of the fossils and the major shifts in climate. Our evidence shows that the Naracoorte giants perished under climatic conditions similar to those under which they previously thrived, which strongly implicates humans in their extinction" said Professor Roberts.
The research project was supported by the South Australian Department for Environment and Heritage, GreenCorp, the Friends of the Naracoorte Caves, the Cave Exploration Group of South Australia, the Commonwealth Natural Heritage Trust Extension Bushcare Program, and the Australian Research Council.
Original PR available via Media Releases (Dec. 22 entry) [Paleontology]
-------
Based on The Geological Society of America's* Geology paper:
"Mammalian responses to Pleistocene climate change in southeastern Australia"
by Gavin J. Prideaux, , Richard G. Roberts, Dirk Megirian, Kira E. Westaway, John C. Hellstrom, Jon M. Olley
Abstract
NYA at: http://dx.doi.org/10.1130/G23070A.1
Resolving faunal responses to Pleistocene climate change is vital for differentiating human impacts from other drivers of ecological change. While 90% of Australia's large mammals were extinct by ca. 45 ka, their responses to glacial-interglacial cycling have remained unknown, due to a lack of rigorous biostratigraphic studies and the rarity of terrestrial climatic records that can be related directly to faunal records. We present an analysis of faunal data from the Naracoorte Caves in southeastern Australia, which are unique not only because of the species richness and time-depth of the assemblages that they contain, but also because this faunal record is directly comparable with a 500 k.y. speleothem-based record of local effective moisture. Our data reveal that, despite significant population fluctuations driven by glacial-interglacial cycling, the species composition of the mammal fauna was essentially stable for 500 k.y. before the late Pleistocene extinctions. Larger species declined during a drier interval between 270 and 220 ka, likely reflecting range contractions away from Naracoorte, but they then recovered locally, persisting well into the late Pleistocene. Because the speleothem record and prior faunal response imply that local conditions should have been favorable for megafauna until at least 30 ka, climate change is unlikely to have been the principal cause of the extinctions.
-------
*About the GSA:
"...Established in 1888, The Geological Society of America provides access to elements that are essential to the professional growth of earth scientists at all levels of expertise and from all sectors: academic, government, business, and industry.
The Geological Society's growing membership unites thousands of earth scientists from every corner of the globe in a common purpose to study the mysteries of our planet and share scientific findings..."
Technorati: mystery, australia, megafauna, palaeontology, paleontology, climate change, journal, geology, ice age, western, australian, museum, flinders, university, humans, fossil, evidence, extinction, geological, society, america, pleistocene, species
Add to: CiteUlike | Connotea | Del.icio.us | Digg | Furl | Newsvine | Reddit | Yahoo
Complexity Constrains Evolution of Human Brain Genes
Despite the explosive growth in size and complexity of the human brain, the pace of evolutionary change among the thousands of genes expressed in brain tissue has actually slowed since the split, millions of years ago, between human and chimpanzee, an international research team reports in the December 26, 2006, issue of the journal, PLOS Biology.
The rapid advance of the human brain, the authors maintain, has not been driven by evolution of protein sequences. The higher complexity of the biochemical network in the brain, they suspect, with multiple gene-gene interactions, places strong constraints on the ability of most brain-related genes to change.
'We found that genes expressed in the human brain have in fact slowed down in their evolution, contrary to some earlier reports,' says study author Chung-I Wu, professor of ecology and evolution at the University of Chicago. 'The more complex the brain, it seems, the more difficult it becomes for brain genes to change. Calibrated against the genomic average, brain-expressed genes in humans appear to have evolved more slowly than in chimpanzee or old-world monkey.'
Humans have an exceptionally big brain relative to their body size. Although humans weigh about 20 percent more than chimpanzees, our closest relative, the human brain weighs 250 percent more. How such a massive morphological change occurred over a relatively short evolutionary time has long puzzled biologists.
Previous reports have argued that the genes that regulate brain development and function evolved much more rapidly in humans than in nonhuman primates and other mammals because of natural selection processes unique to the human lineage.
The comparative pace of organ-specific evolution, however, turns out to be difficult to measure. To assess the speed with which both humans and chimpanzees accumulated many small differences in gene sequences accurately, Wu and colleagues in Taiwan and Japan decided to sequence several thousand genes expressed in the brain of the macaque monkey and compare them with available genomic sequences from human, chimpanzee, and mice.
What they found was that the "more advanced" species had faster overall rates of evolution. So, on average, the genes from humans and chimpanzees changed faster than genes from monkeys, which changed faster than those from mice.
They explained the trend as a correlate of smaller population size in the more advanced species. Species with smaller population size can more easily escape the harsh scrutiny of natural selection.
When they compared the pace of evolution among genes expressed in the brain, however, the order was reversed. When calibrated against the genomic average, brain genes in humans evolved more slowly than in other primates, which were slower than mice.
"We would expect positive selection to work most effectively on tissue-specific genes, where there would be fewer conflicting requirements," says Wu. "For example, genes expressed only in male reproductive tissues have evolved very rapidly."
Brains, however, "are intriguing in this respect," Wu says. Genes that are expressed only in the brain evolved more slowly than those that are expressed in the brain as well as other tissues, and those genes evolved more slowly than genes expressed throughout the rest of the organism.
The authors attribute the slowdown to mounting complexity of interactions within the brain. "We know that proteins with more interacting partners evolve more slowly," Wu said. "Mutations that disrupt existing interactions aren't tolerated."
Although the gene sequences from human and chimpanzee remain very similar, previous studies in tissues other than the brain have shown that gene expression varies widely. Other studies have found that, within the brain, the abundance of expressed genes per neuron appears to be greater in humans.
"On the basis of individual neurons of the brain, humans may indeed have a far more active, or even more complex, transcription profile than chimpanzee," the authors note. "We suggest that such abundant and complex transcription may increase gene-gene interactions and constrains coding-sequence evolution."
Future studies of brain function and evolution will increasingly take advantage of the approaches of systems biology, Wu suggested. "The slowdown in genetic evolution in the more advanced organs makes sense," he said, "only when one takes a systems perspective."
Academia Sinica and the National Sciences Council of Taiwan, the Ministry of Health and Welfare of Japan, and the U.S. National Institutes of Health funded the research. Additional authors are C.-K. James Shen, Hurng-Yi Wang and Huan-Chieh Chien of Academia Sinica, Taiwan; Naoki Osada and Katsuyuki Hashimoto of the National Institute of Infectious Diseases, Japan; Sumio Sugano of the University of Tokyo, Japan; Takashi Gojobori of the National Institute of Genetics, Japan; Chen-Kung Chou of Taipei Veterans General Hospital, Taiwan; and Shih-Feng Tsai of the National Health Research Institute, Taiwan.
Source: University of Chicago News Office
-------
Based on the open access/free PLoS Biology article:
Rate of Evolution in Brain-Expressed Genes in Humans and Other Primates
Citation: Wang HY, Chien HC, Osada N, Hashimoto K, Sugano S, et al. (2007) Rate of Evolution in Brain-Expressed Genes in Humans and Other Primates. PLoS Biol 5(2): e13 DOI: 10.1371/journal.pbio.0050013
Abstract
Brain-expressed genes are known to evolve slowly in mammals. Nevertheless, since brains of higher primates have evolved rapidly, one might expect acceleration in DNA sequence evolution in their brain-expressed genes. In this study, we carried out full-length cDNA sequencing on the brain transcriptome of an Old World monkey (OWM) and then conducted three-way comparisons among (i) mouse, OWM, and human, and (ii) OWM, chimpanzee, and human. Although brain-expressed genes indeed appear to evolve more rapidly in species with more advanced brains (apes > OWM > mouse), a similar lineage effect is observable for most other genes. The broad inclusion of genes in the reference set to represent the genomic average is therefore critical to this type of analysis. Calibrated against the genomic average, the rate of evolution among brain-expressed genes is probably lower (or at most equal) in humans than in chimpanzee and OWM. Interestingly, the trend of slow evolution in coding sequence is no less pronounced among brain-specific genes, vis-à-vis brain-expressed genes in general. The human brain may thus differ from those of our close relatives in two opposite directions: (i) faster evolution in gene expression, and (ii) a likely slowdown in the evolution of protein sequences. Possible explanations and hypotheses are discussed.
Author Summary
Whether comparing morphology or cognitive ability, it is clear that the human brain has evolved rapidly relative to that of other primates. But the extent to which genes expressed in the brain also reflect this overall pattern is unclear. To address this question, it's necessary to measure any variations in the DNA sequences of these genes between human and chimpanzee. And, to do this as accurately as possible, it's also important to require an appropriate reference group to act as a benchmark against which the differences can be measured. We therefore compared publicly available genomic sequences of chimps and humans with complementary DNA sequences of several thousand genes expressed in the brain of another closely related primate - the macaque, an Old World monkey - as well as the more distantly related mouse. Our analyses of the rates of protein evolution in these species suggest that genes expressed in the human brain have in fact slowed down in their evolution since the split between human and chimpanzee, contrary to some previously published reports. We suggest that advanced brains are driven primarily by the increasing complexity in the network of gene interactions. As a result, brain-expressed genes are constrained in their sequence evolution, although their expression levels may change rapidly.
Technorati: complexity, human, brain, chimpanzees, research, plos, biology, genes, evolution, ecology, chicago, monkey, primates, dna, expression, morphology, cognitive, ability, old, world
Add to: CiteUlike | Connotea | Del.icio.us | Digg | Furl | Newsvine | Reddit | Yahoo
Sunday, December 24, 2006
Predator Mimicry: Metalmark Moths Mimic Their Jumping Spider Predators
An open access/free paper (includes video) from PLoS ONE:
Predator Mimicry: Metalmark Moths Mimic Their Jumping Spider Predators
by Jadranka Rota (info), David L. Wagner* (info)
Abstract
Cases of mimicry provide many of the nature's most convincing examples of natural selection. Here we report evidence for a case of predator mimicry in which metalmark moths in the genus Brenthia mimic jumping spiders, one of their predators. In controlled trials, Brenthia had higher survival rates than other similarly sized moths in the presence of jumping spiders and jumping spiders responded to Brenthia with territorial displays, indicating that Brenthia were sometimes mistaken for jumping spiders, and not recognized as prey. Our experimental results and a review of wing patterns of other insects indicate that jumping spider mimicry is more widespread than heretofore appreciated, and that jumping spiders are probably an important selective pressure shaping the evolution of diurnal insects that perch on vegetation.
Introduction
The phenomenon of mimicry, a high degree of resemblance due to selection, was first proposed in 1862 by Sir Walter Henry Bates (info) upon his return from eleven years as a professional collector in Amazon. Writing about butterfly wing patterns, Bates noted, "... on these expanded membranes Nature writes, as on a tablet, the story of the modifications of species..." Bates proposed that longwings and other butterflies gain protection by mimicking distasteful species and that the resemblances among such unrelated insects lent support to Charles Darwin's newly proposed theory of natural selection [1]. Since Bates's initial contribution various cases of mimicry have been identified from across the tree of life. In this paper, we describe a curious form of Batesian mimicry - again involving the wing patterns of Lepidoptera - in which prey (metalmark moths) obtain protection by mimicking their predators (jumping spiders)
Many examples of Batesian and Mullerian mimicry and camouflage have been described [1]–[4]. Even cases of aggressive mimicry, where predators mimic prey, are known (e.g., females of Photuris fireflies lure males of different firefly species to their death by mimicking their courtship signals [5]). However, predator mimicry - cases in which prey have evolved to mimic their predators to thwart predation attempts - are both exceptional and rare.
Predator mimicry was suggested for owls, where owl ear tufts mimic mammalian predators for protection from such predators as lynx, fox, and marten [6]. Another potential case of predator mimicry is among South American cichlids: coloration and spotting of certain prey species makes them so similar to their predators that they are thought to be their mimics [7]. Eyespots on the wings of giant silk moths and other Lepidoptera undoubtedly mimic eyes of mammalian predators - but here the eyes may function not to mimic their would-be predators, but to resemble a much larger animal, one sizable enough to be a threat to lepidopteran would-be predators. Hence, these eyespots might be regarded as startle coloration [8]. However, in none of these cases are there experimental data demonstrating the efficacy of this mimicry. Our literature review suggests there are few well-supported cases of predator mimicry: e.g., lycaenid butterflies that chemically mimic ants [9] and salticid spiders that mimic ants to avoid being preyed upon by them [10] (other ant mimics probably gain protection from all predators that tend to avoid ants [11]). Here we present evidence for another case of predator mimicry involving salticid spiders, but in this case salticids are predators and not prey.
Continued at "Predator Mimicry: Metalmark Moths Mimic Their Jumping Spider Predators"
Citation: Rota J, Wagner DL (2006) Predator Mimicry: Metalmark Moths Mimic Their Jumping Spider Predators. PLoS ONE 1(1): e45. doi:10.1371/journal.pone.0000045
-------
See The Arts of Deception: Mimicry and Camouflage:
"There are three forms of mimicry utilized by both predator and prey: Batesian mimicry, Muellerian mimicry, and self-mimicry. Mimicry refers to the similarities between animal species; camouflage refers to an animal species resembling an inanimate object..."
-------
*David Wagner is author of "Caterpillars of Eastern North America : A Guide to Identification and Natural History"
From the Preface:
"I recently attended a seminar at Harvard University to hear Stefan Cover speak. He started off simply enough. "Everyone needs an obsession. Mine is ants." Everyone chuckled … more than a few heads nodded in agreement. For the past ten years mine has been caterpillars. They have provided a bounty of trip memories, abundant photographic opportunities, led to dozens of collaborations and friendships, some of which will be lifelong, and introduced me to a world full of beauty, change, carnage, and discovery. Stefan was right.
My goal in writing this guide is twofold. First, to provide larval images and biological summaries for the larger, commonly encountered caterpillars found east of the 100th meridian. Sounds simple, yet the problems associated with compiling such information are legion: literature is scattered, lacking, or, worse, especially in the case of some early accounts, wrong. For many common moths the species taxonomy is still under study, life histories are incompletely known, and distributional data have yet to be assembled. In this guide I offer a synopsis for each species that includes information on its distribution, phenology, and life history.
...Behaviors and phenomena previously believed to be exceptional or uncommon are shown to be otherwise: e.g., both Batesian and Mullerian mimicry appear to be more prevalent in caterpillars than previously recognized. Pronounced developmental changes (in form, coloration, and behavior), bordering on hypermetamorphosis, were seen in several families - striking examples occur among the daggers and slug caterpillars..."
Technorati: predators, mimicry, video, spiders, natural selection, insects, evolution, theory, charles darwin, tree, life, batesian, mullerian, butterflies, ants, self, caterpillars, discovery, taxonomy
Add to: CiteUlike | Connotea | Del.icio.us | Digg | Furl | Newsvine | Reddit | Yahoo
'The Only One in Step' by Richard Dawkins
'The Only One in Step' by Richard Dawkins (The God Delusion: Amazon Astore UK | US):
I can't find the original volume so I may have got the exact words wrong, but I recall one of those marvellous old Punch cartoons in which every last detail is painstakingly explained. A devoted mother is looking proudly on at a military parade as her son's platoon marches past: 'There's my boy, he's the only one in step!' On The Guardian letters page of December 19th 2006, I initiated an exchange about Professor Andrew McIntosh* (info) of Leeds university, who has publicly stated that he believes the world is only 6,000 years old, and publicly stated that the theory of evolution violates the second law of thermodynamics**. Both these beliefs place McIntosh out of step with his scientific colleagues, not just his platoon but the entire regiment - to paraphrase Evelyn Waugh, the whole ruddy division. Amazingly, McIntosh is Professor of Thermodynamics at Leeds, and, equally amazingly, a letter supporting him has now appeared from Professor Stuart Burgess (homepage), Head of the Department of Mechanical Engineering at Bristol University . Other letters to the Editor indicate that a distressing number of otherwise knowledgeable and intelligent people have little conception of the enormity of what is being said.
Science doesn't work by vote and it doesn't work by authority. It is possible that Burgess and McIntosh really are the only ones in step, and the whole scientific establishment is flat wrong. Indeed, I shall bias my discussion in their favour by continuing to use that word 'establishment' with all its pejorative overtones of fuddyduddy, stick-in-the-muddy authoritarianism. I like mavericks. I like free spirits who buck the trend and strike out on their own. They are not usually right, but on the rare occasions when they are, they are very right indeed: importantly so, and all power to them. Maybe Burgess and McIntosh are right and all the rest of us – biologists, geologists, archeologists, historians, chemists, physicists, cosmologists and, yes, thermodynamicists and respectable theologians, the vast majority of Nobel Prizewinners, Fellows of the Royal Society and of the National Academies of the world - are wrong. Not just slightly wrong but catastrophically, appallingly, devastatingly wrong. It is possible, and I am going to follow that possibility through to its logical conclusion. I shall not here defend the views held by the scientific establishment. I am among those who have done that elsewhere, in many books. My purpose in this article is only to convey the full magnitude of the error into which, if Burgess and McIntosh are right, the scientific establishment has fallen.
Continued at "The Only One in Step"
--------
*On November 29th 2006, Leeds University (UK) issued the following press release:
Professor Andrew McIntosh's directorship of Truth in Science***, and his promotion of that organisation's views, are unconnected to his teaching or research at the University of Leeds in his role as a professor of thermodynamics. As an academic institution, the University wishes to distance itself publicly from theories of creationism and so-called intelligent design which cannot be verified by evidence.
--------
**Info on the Second Law of Thermodynamics:
Sometimes people say that life violates the second law of thermodynamics. This is not the case; we know of nothing in the universe that violates that law. So why do people say that life violates the second law of thermodynamics? What is the second law of thermodynamics?
The second law is a straightforward law of physics with the consequence that, in a closed system, you can't finish any real physical process with as much useful energy as you had to start with - some is always wasted. This means that a perpetual motion machine is impossible. The second law was formulated after nineteenth century engineers noticed that heat cannot pass from a colder body to a warmer body by itself.
According to philosopher of science Thomas Kuhn, the second law was first put into words by two scientists, Rudolph Clausius and William Thomson (Lord Kelvin), using different examples, in 1850-51 (2). American quantum physicist Richard P. Feynman, however, says the French physicist Sadi Carnot discovered the second law 25 years earlier (3). That would have been before the first law, conservation of energy, was discovered! In any case, modern scientists completely agree about the above principles.
-------
***From the Truth in Science website:
"Welcome to Truth in Science, a new organisation to promote good science education in the UK. Our initial focus will be on the origin of life and its diversity.
For many years, much of what has been taught in school science lessons about the origin of the living world has been dogmatic and imbalanced. The theory of Darwinian evolution has been presented as scientifically uncontroversial and the only credible explanation of origins."
Technorati: richard dawkins, god delusion, the guardian, theory, evolution, truth, science, second, law, thermodynamics, creationism, intelligent design, origins, life
Add to: CiteUlike | Connotea | Del.icio.us | Digg | Furl | Newsvine | Reddit | Yahoo